Abstract:
Living organisms at various length scales exhibit a wide range of complex movements driven by coordinated expansion and contraction of tissues. Drawing inspiration from them, we investigate protein-based soft actuators, leveraging their unique combination of biocompatibility and tunable mesoscale structures, which include both amorphous and crystalline forms. Annealing the actuator to vary the degree of crystallinity between 20% and 45%, we control the speed and extent of actuation in response to vapor stimuli (see Figure 1). Notably, the actuator with higher crystallinity exhibits reduced responsiveness to the stimuli. To further investigate the observed phenomenon, we measure the swelling kinetics of thin protein films using ultra-fast synchrotron X- ray reflectivity at the PF-AR Beamline NE-7A, Photon Factory in Japan. Our analysis reveals that the un-annealed films swell more than the annealed ones, a difference attributed to variations in their intrinsic mesoscale structure. Our study unambiguously establishes a direct link between protein mesoscale structure and vapor sorption behavior, offering insights into bioinspired actuation. These findings will be discussed in detail.
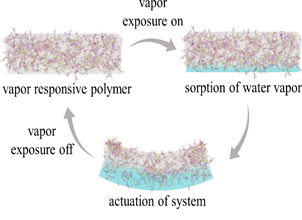
Figure 1. Schematic shows the vapor-response actuation of protein films
Biography:
Miss. Sarah Ahmad Siraj is a final year Ph.D. student at IITM. Her masters was in physics with her thesis focusing on the sensing of various gases using BaTiO3. Currently she’s working in the field of soft matter focusing on vapor-responsive protein based soft actuators. Furthermore, she is also involved with synchrotron reflectivity and scattering studies of thin nm protein films to study the swelling kinetics in detail.
Copyright 2024 Mathews International LLC All Rights Reserved